
Leading Providers of Compounding Chemicals, Compounding Supplies, Pharmacist Training, Compound Formulas, Compounding Software,












The annual Compounding Pharmacy Compliance conference is back, offering a deep dive into key compliance areas and strategies to ensure inspection readiness in an evolving regulatory landscape. As USP regulations and FDA oversight continue to evolve in 2025, staying informed is critical to maintaining high-quality compounding standards, improving patient safety, and ensuring compliance. This conference is designed to help compounding professionals navigate regulatory updates, address compliance challenges, and optimize operational practices for both 503A and 503B facilities.
What’s News in 2025?
Key program features this year include 2 days of live content from 30+ industry thought leaders, live speaker Q&A, breakout discussions and on-demand content. Including perspectives from the Acacia Apothecary Wellness, Leiters Health, Alliance for Pharmacy Compounding, Wells Pharmacy Network, SandsRX Pharmacy, Outsourcing Facilities Association, Olympia Pharmaceuticals, Farmakeio Pharmacy Network, Revelation Pharma, Town & Country Compounding, and more, this event is not to be missed.
This year’s program includes advanced insights from expert perspectives, including the opportunity to:
Choose Your Learning Path with Tracked Programming!
503A Track
a. Navigating UP Regulatory Adherence & 503A-Specific Strategies to Operationalize
b. Managing Drug Shortages – Compliance Strategies for 503A Pharmacies
c. Navigating Drug Shortages for Health Systems – Collaborative Strategies with 503A Pharmacies
d. Mastering State Board 503A Requirements and Enhancing Inspection Readiness
503B Track
a. Examining the 503B Regulatory Landscape – Recent Updates and Industry Impact
b. Legal Spotlight – FDA’s Removal of Tirzepatide from the Drug Shortage List
c. Mastering Drug Shortages – Regulatory and Operational Strategies for 503Bs
d. Best Practices in 503B Quality Assurance to Ensure cGMP Compliance
And more!
For full program details and registration for you and your team, visit: https://informaconnect.com/compounding-pharmacy-compliance/.




The Voice for Pharmacy Compounding
APC represents compounding pharmacists and technicians in both 503A and 503B settings, as well as prescribers, educators, patients, and suppliers.
Our passion for compounding is fueled by our members’ passion for their patients — and the medications they create. APC is focused on strategies to assure that millions of patients can continue to access those compounded medications.
Our commitment — not only to our dues-paying members but also to their patients — is to lead, to influence, to speak, and to serve so that the practice of pharmacy compounding is not merely preserved but is elevated as a key component of health care delivery for millions of patients across America.
APC members subscribe to the Pharmacy Compounders Code of Ethics, comprised of 10 tenets that guide their professional, clinical, and business practices.
APC members play a vital role in the American healthcare system and in individual patients’ lives, preparing essential personalized medications for a range of issues, including autism, oncology, dermatology, ophthalmology, pediatrics, women’s health, and more.
Through its ally grassroots organization, the Partnership for Personalized Prescriptions, APC represents more than 150,000 patients, compounding professionals, prescribers, and others.
APC is committed to ensuring the rights of physicians to prescribe, of pharmacists to prepare, and of patients to take personalized medication solutions to meet their unique health care needs.
• Visit the Alliance for Pharmacy Compounding Website
• View the Alliance for Pharmacy Compounding Platinum Pages Publication Ad
• View the Alliance for Pharmacy Compounding 20Ways Publication Profile
• Print the Alliance for Pharmacy Compounding Booth Information




Associates of Cape Cod, Inc. (ACC), is one of the world's largest manufacturers of products developed to detect and quantify gram-negative bacterial endotoxins and (1-3)-ß-D-glucans. Our products are used worldwide by leading pharmaceutical and medical device companies to ensure the safety of their parenteral drugs, biological products and medical devices. Our goal is to provide the best products and services, as well as the best technical support and customer service, in our industry to enhance the productivity and efficiency of all our customers. We are ISO 13485: 2003 certified, our laboratories are FDA Inspected and DEA Licensed and our Beacon Diagnostics ® laboratory is CLIA certified.
ACC is one of the world's largest manufacturers of products developed to detect and quantify gram-negative bacterial endotoxins and (1-3)-ß-D-glucans. Our products are used worldwide by leading pharmaceutical and medical device companies to ensure the safety of their parenteral drugs, biological products, and medical devices. Our Customer Service, Technical Service, and Contract Testing teams are ready to assist you with all aspects of endotoxin and glucan testing.
Pyroclear® Accessory Products
Pyroclear Pyroclear® brand accessory products are the first products in the industry that are certified to be free of interfering endotoxin and (1-3)-ß-D-glucan contamination. Pyroclear brand certified products include depyrogenated test tubes, 96-well microplates, pipette tips and LAL Reagent water. These products are designed to reduce Out of Specification (OOS) investigations due to contaminated accessories.
ACC is proud to offer a variety of instrumentation to support all your testing needs. Instruments like our Pyros Kinetix® incubating kinetic tube reader which offers the highest turbidimetric assay sensitivity in the industry. Bridge the gap between the affordability of filter-based readers and flexibility of monochromator-based systems with our VersaMax™ microplate reader.
ACC offers a complete suite of reliable, easy to use research products. Developed for research purposes only, ACC's Research Products are designed to improve pyrogen testing while offering our high standard of effectiveness.
Precise, easy-to-interpret data is an essential part of kinetic endotoxin detection programs. ACC offers three software packages designed to improve the efficiency and the compliance of your testing program all featuring 21 CFR Part 11 compliance, dynamic reporting options and increased data security.
• Visit the Associates of Cape Cod Inc Website
• View the Associates of Cape Cod Inc Literature
• View the Associates of Cape Cod Inc Platinum Pages Publication Ad
• View the Associates of Cape Cod Inc 20Ways Publication Profile







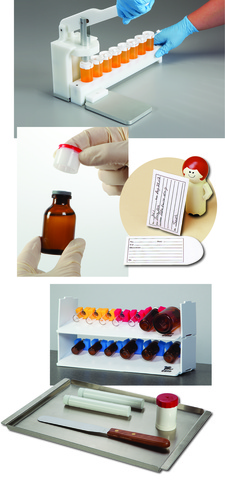
Pharmacy Compounding Supplies
The HCL Compounding and Dispensing line features a diverse selection of products with a special focus on USP 797 & 795 supplies as well as those specially designed for precise medication measuring, mixing and filling. We’re committed to offering products that help you increase productivity, maximize efficiency and improve accuracy in all aspects of your business.
To eliminate hand fatigue when packaging liquid compounds, try our Liquid Unit Dose (LUD) Press. It has an aluminum base and rod that easily seals caps onto vials or cups. Choose a heavy-duty Holder for Vials or a Holder for Cups, each of which will hold up to eight items side by side to prevent spills. A raised notch on the LUD Press platform helps guide your holder into position to create a secure seal on every vial or cup, every time. You spend less time on alignment and improve productivity.
We create metal products to match your unique specifications so you can work more efficiently and produce better results. Could your facility benefit from stainless steel storage containers, lock boxes and utility trays to simplify USP <797> compliance? Do you have an existing product you'd like to upgrade with a specially sized metal adaptor such as a bracket, plate, clamp or hanger that is USP <797> friendly? Whatever solution you seek, HCL can deliver. Our designers use stainless steel and aluminum, dependent upon your needs, and our design consultation services are always FREE!
• Visit the Health Care Logistics Website
• View the Health Care Logistics Platinum Pages Publication Ad



The International Journal of Pharmaceutical Compounding (IJPC) is a bi-monthly, scientific and professional digital journal emphasizing quality pharmaceutical compounding. IJPC is the only publication that covers pharmaceutical compounding topics relevant and necessary to empower pharmacists to meet the needs of today's patients. No other publication features hands-on, how-to compounding techniques or the information that contemporary pharmacists need to provide individualized care.
Each issue includes peer-reviewed scientific articles, articles on pertinent topics, informational and professional articles, calculations, and other departments relevant to the practice of compounding. Articles and manuscripts are reviewed by pharmacy practitioners, academicians and scientists, as well as by others with expertise in various specialties.
The International Journal of Pharmaceutical Compounding is indexed by the International Pharmaceutical Abstracts,the Cumulative Index to Nursing & Allied Health Literature print index and database, the Chemical Abstracts Service, Elsevier Bibliographic Databases including EMBASE, EMNursing, Compendex, GEOBASE, Mosby Yearbooks and Scopus, the world’s largest abstract and indexing database of scientific and social sciences literature with more than 8 million users at more than 500 institutions worldwide.
Manuscripts published in the International Journal of Pharmaceutical Compounding are read and researched worldwide.
IJPC has been recognized with the following awards:
Please contact us by phone at 800-757-4572 or click on the link below to visit our website.
• View the IJPC Platinum Pages Publication Ad



International Medical Industries
Prep-Lock Tamper Evident Caps Deter Drug Diversion...














Connect with thousands of pharmacy professionals throughout every practice setting.
The server is experiencing a problem at this time. Try again later.

























































