




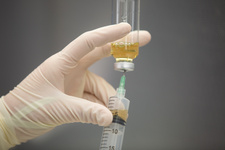
CriticalPoint strives on increasing patient safety through a variety of educational offerings focusing on UPS 795, 797, and 800. Partnering with industry experts, we provide the most-current and engaging training on industry standards resulting in improved competency and patient safety.
Our mission is helping customers achieve a measurable state of excellence through trustworthy, time-tested, and practical compounding and patient-safety solutions.
Our vision is to advance compounding practices through leadership and delivery of evidence-based, best practice compounding, and patient-safety solutions.
Our course in Sterile Compounding consists of 32 eLessons (worth 34.5 hours of ACPE-approved CE for Pharmacist or Technicians). These eLessons are specifically designed to teach and reinforce the skills required to meet the requirements of USP 797 and 800. Visit our website to view a complete listing of lessons and learning objectives.
CriticalPoint has expanded our eLearning offerings to include Nonsterile Compounding. The course Best Practices and Compliance for USP 795 for Nonsterile Compounding is written by expert instructors and includes 11 eLessons and post tests covering all aspects of USP 795 (proposed revisions). The course is worth 11 hours of ACPE-approved CE. Visit our website to learn more about the course.
This course develops fundamental math skills during 8 eLessons that incorporate practical examples from pharmacy work settings. This offering may benefit certain pharmacists, technicians and students who need to build a sound math skills framework, essential to successful pharmacy practice.
The web-based curriculum will ensure participants acquire a comprehensive understanding of how and why 503B practice evolved. These eLessons also summarize each of the FDA draft and final guidances so that learners know where to seek guidance for 503B practice.
Save money and avoid time away from work and your family! Receive engaging, detailed, and practical lectures and discussions virtually.
Detailed standard operating procedures are the foundation of robust sterile and nonsterile compounding practice. When written properly, they should tell the reader exactly what to do as well as why, where, when and how to perform the activity.
CriticalPoint has developed of a set of template policies, procedures, and accompanying documentation forms (together referred to as the SOPs). They are effective in establishing robust operating procedures that ensure patient safety.
These detailed procedures and forms reflect not only guidance to foster achievement of compliance with Chapter 795, 797, and 800 but with aseptic processing best practices as well. They are continually refined and updated to reflect regulatory changes (like Chapter 797 and Chapter 800 revisions). They are consistent with the CriticalPoint eLearning Training while providing more specific “how to” detail in regard to process as well as documentation. The SOPs can be harmonized to match the actual working conditions of your compounding facility and practice.
 RXinsider | The preceding content is a booth exhibit in
RXinsider's Virtual Pharmacy Trade Show.
RXinsider | The preceding content is a booth exhibit in
RXinsider's Virtual Pharmacy Trade Show.
Share booth #16117 with others.